Abstract
Background and Scientific Rationale:
Multiple myeloma (MM) is often preceded by monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). However, the current standard of care for MGUS and SMM is observation.
Studies have found that patients with plasma cell disorders have gut dysbiosis. Plant-based diets, probiotics, omega 3, and curcumin supplements have previously been shown to alter the gut microbiome and increase stool butyrate. Butyrate is a short-chain fatty acid (SCFA) that inhibits histone deacetylases and NF-κB resulting in reduced formation of pro-inflammatory cytokines. It, therefore, has anti-cancer and anti-inflammatory properties. Therefore, a dietary/supplement intervention will be studied in patients with SMM.
This is a two-week randomized study to evaluate the effects of a whole-food plant-based diet (WFPBD), probiotics, omega 3, and curcumin supplements on the gut microbiome and stool butyrate levels compared to baseline in SMM patients. This study builds on our prior NUTRIVENTION pilot study (NCT04920084). To our knowledge, this is the first telehealth national nutrition intervention study design with a microbiome endpoint.
Study Design and Methods:
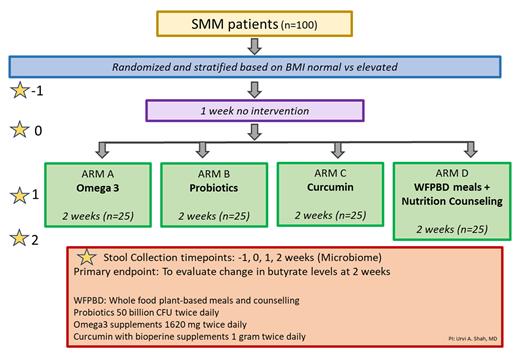
This is a randomized, non-blinded, telehealth-based, remote, national pilot study with 100 patients in 4 arms (25 per arm) in partnership with HealthTree Foundation (Figure 1). Randomization will be stratified based on BMI (normal/elevated).
Study Population:
100 SMM patients over the age of 18 years will be enrolled and randomized to one of four arms (25 patients per arm).
Inclusion Criteria:
ECOG performance status 0-3
Willingness to comply with study
Interested in learning to cook plant-based recipes
Access to smart mobile phone or device
Be residing within the United States for the study duration
Exclusion Criteria:
Supplement intake (other than those needed for a medical indication) must be reviewed by PI before enrollment. If patient is on a supplement (including curcumin, probiotic, omega 3) they must stop these for 2 weeks prior to enrollment on study.
Legume allergy
Severe allergies such as anaphylactic shock to peanuts
Concurrent participation in weight loss/dietary trials or defined programs (that require specified diets/supplements)
Study Treatment:
Patients will be randomized to one of four arms and will receive a whole-foods plant-based diet with nutrition counseling (via Plantable) or probiotics (via Vita Miracle) or omega-3 (via QWell pharmaceuticals) or curcumin (via Sabinsa pharmaceuticals) supplements twice daily for 2 weeks (Figure 1). The supplements will be provided through the WellRabbit platform. Data on dietary intake will be collected for all participants using a dietary monitoring app, Keenoa. Dietary adherence will be assessed by a research dietitian. Patients will participate in study visits remotely.
Objectives:
Primary
Evaluate change in stool butyrate levels on a plant-based diet, curcumin, omega 3, and probiotic intervention at 2 weeks when compared to baseline.
Secondary and Exploratory objectives include change in stool butyrate at 1 week, compliance at 2 weeks, baseline dietary patterns, other changes in the gut microbiome, and racial differences in the microbiome.
Statistical Methods:
The primary endpoint is change in stool butyrate levels at 2 weeks when compared to baseline (average of -1- and 0-week samples). Based on previous and internal studies, we expect the study to be adequately powered to detect a difference of ≥20% from baseline to the end of the intervention. We will call the intervention promising if we detect an increase in stool butyrate levels at 2 weeks (i.e., one-sided p-value <0.05) when compared with baseline.
Disclosures
van den Brink:Rheos Medicines: Honoraria; Vor Biopharma: Honoraria; Pluto Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Notch Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Seres Therapeutics: Current holder of stock options in a privately-held company, Honoraria, Other: IP Licensing , Research Funding; Frazier Healthcare Partners: Honoraria; Nektar Therapeutics: Honoraria; Ceramedix: Honoraria; Lygenesis: Honoraria; GlaskoSmithKline: Honoraria; Da Volterra: Honoraria; Thymofox: Honoraria; Garuda: Honoraria; Novartis (Spouse): Honoraria; Synthekine (Spouse): Honoraria; Beigene (Spouse): Honoraria; Kite (Spouse): Honoraria; Juno Therapeutics: Other: IP Licensing ; DKMS: Other: fiduciary role on the Foundation Board ; Wolters Kluwer: Patents & Royalties. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding. Ahlstrom:Pfizer, BMS, Janssen, Takeda Oncology, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lesokhin:Sanofi: Research Funding; Janssen, Pfizer, Iteos, Sanofi, Genmab: Honoraria; Janssen, Pfizer, BMS, Genentech/Roche: Research Funding; Serametrix, inc: Patents & Royalties; Trillium Therapeutics: Consultancy, Research Funding; Pfizer, Genmab, Sanofi, Iteos, BMS, Janssen: Consultancy; BMS: Honoraria; Amgen: Honoraria; Memorial Sloan Kettering Cancer Center: Current Employment. Shah:ACCC: Honoraria; Janssen: Consultancy, Research Funding; MashUpMD: Honoraria; MJH Lifesciences: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Research Funding; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal